| Structure | Name/CAS No. | Articles |
|---|---|---|
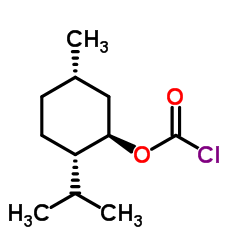 |
(-)-MENTHYL CHLOROFORMATE
CAS:14602-86-9 |
|
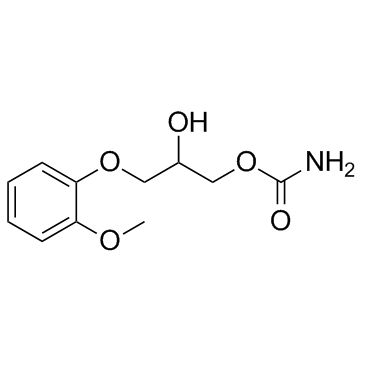 |
methocarbamol
CAS:532-03-6 |