| Structure | Name/CAS No. | Articles |
|---|---|---|
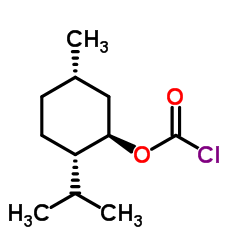 |
(-)-MENTHYL CHLOROFORMATE
CAS:14602-86-9 |
Saumen Hajra, Manishabrata Bhowmick, Biswajit Maji, Debarshi Sinha
Index: J. Org. Chem. 72 , 4872, (2007)
Full Text: HTML
New chiral N-chloroimidodicarbonates, which function as efficient chiral chlorinating agents, were designed and synthesized. Among these, C(2)-symmetric (1R,2S,5R)-(-)-menthyl-N-chloroimidodicarbonate 2a provided moderate to good enantioselectivity (up to 40%) for the chlorination of silyl enol ethers to afford alpha-chloroketones only in the presence of a suitable Lewis acid such as Sm(OTf)(3).
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
(-)-MENTHYL CHLOROFORMATE
CAS:14602-86-9 |
C11H19ClO2 |
|
Stereospecific liquid chromatographic analysis of racemic ad...
[J. Chromatogr. A. 493(2) , 402-8, (1989)] |
|
Resolution of antihypertensive aryloxypropanolamine enantiom...
[J. Chromatogr. A. 487(1) , 197-203, (1989)] |
|
Detection of amphetamine and methamphetamine in urine by gas...
1991-01-01 [J. Anal. Toxicol. 15(5) , 256-9, (1991)] |
|
Chirality and the origin of life: in situ enantiomeric separ...
2002-06-01 [Chirality 14(6) , 527-32, (2002)] |
|
N-Menthoxycarbonylation combined with trimethylsilylation fo...
2006-01-20 [J. Chromatogr. A. 1103(1) , 177-81, (2006)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved