Phosphoramidate derivatives of 2',5'-dideoxyadenosine as potential inhibitors of the EDHF phenomenon.
F Gavazza, F Daverio, A T Chaytor, T M Griffith, C McGuigan
Index: Nucleosides Nucleotides Nucleic Acids 24(5-7) , 553-5, (2005)
Full Text: HTML
Abstract
P-site inhibitors of adenyl cyclase, such as the dideoxynucleosides 2',3'-ddA and 2',5-ddA, have been shown to attenuate EDHF phenomenon in rabbit arteries and veins. In order to present the dideoxynucleosides as pre-activated nucleotides and bypass the kinase, as well as to prevent their metabolism to dideoxyinosine by adenosine deaminase, the aryloxyphosphoramidate approach has been successfully applied, initially on the 2',3'-ddA. In the present work a new series of 2',5'-ddA phosphoramidates has been synthesized, representing the first example of phosphoramidate protide not at the 5'-position.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
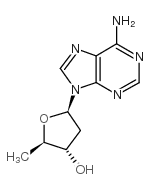 |
2',5'-Dideoxyadenosine
CAS:6698-26-6 |
C10H13N5O2 |
|
Involvement of cAMP in nerve growth factor-triggered p35/Cdk...
2010-08-01 [Am. J. Physiol. Cell Physiol. 299 , C516-27, (2010)] |
|
A new site and mechanism of action for the widely used adeny...
2013-01-01 [Mol. Pharmacol. 83(1) , 95-105, (2013)] |
|
Identification of 17,20β,21-trihydroxy-4-pregnen-3-one (20β-...
2011-02-01 [Gen. Comp. Endocrinol. 170(3) , 629-39, (2011)] |
|
Homotypic gap junctional communication associated with metas...
2010-12-01 [Cancer Res. 70 , 10002-11, (2010)] |
|
Role of regulator of G-protein signaling 2 (RGS2) in periodo...
2007-01-01 [Cell Biochem. Funct. 25(6) , 753-8, (2007)] |