Nucleosides, Nucleotides & Nucleic Acids
2009-05-01
Studies on the glycosylation of pyrrolo[2,3-d] pyrimidines with 1-O-acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose: the formation of regioisomers during toyocamycin and 7-deazainosine syntheses.
Peter Leonard, Sachin A Ingale, Ping Ding, Xin Ming, Frank Seela
Index: Nucleosides Nucleotides Nucleic Acids 28(5) , 678-94, (2009)
Full Text: HTML
Abstract
Glycosylation of silylated 4-amino-6-bromo-5-cyano-7H-pyrrolo[2,3-d]pyrimidine (9) with 1-O-acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose (10) under "one-pot" glycosylation conditions (MeCN, TMSOTf) yielded the N-7 isomer 11 together with the N-1 compound 13 (ratio = 2:1). When the same conditions were applied to 4-hydroxy-7H-pyrrolo[2,3-d]pyrimidine (21) the N-3 isomer 22 was the only glycosylation product formed in almost quantitative yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
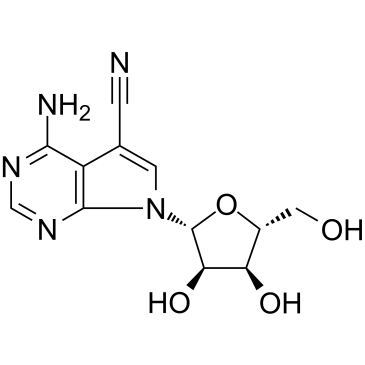 |
Toyocamycin
CAS:606-58-6 |
C12H13N5O4 |
Related Articles:
More...
|
Synthesis of pyrrolo[2,1-f][1,2,4]triazine C-nucleosides. Is...
2001-03-09 [Carbohydr. Res. 331(1) , 77-82, (2001)] |
|
Synthesis of 2'-beta-C-methyl toyocamycin and sangivamycin a...
2005-02-01 [Bioorg. Med. Chem. Lett. 15(3) , 725-7, (2005)] |
|
Cell cycle arrest and cytochrome c-mediated apoptotic induct...
2010-02-01 [J. Microbiol. Biotechnol. 20(2) , 433-7, (2010)] |
|
Synthesis of carbocyclic analogs of 2',3'-dideoxysangivamyci...
2001-01-01 [Nucleosides Nucleotides Nucleic Acids 20(10-11) , 1823-30, (2001)] |
|
Deciphering deazapurine biosynthesis: pathway for pyrrolopyr...
2008-08-25 [Chem. Biol. 15(8) , 790-8, (2008)] |