Kinetics, mechanism, and computational studies of rhenium-catalyzed desulfurization reactions of thiiranes (thioepoxides).
Abdellatif Ibdah, William S Jenks, James H Espenson
Index: Inorg. Chem. 45(14) , 5351-7, (2006)
Full Text: HTML
Abstract
The oxorhenium(V) dimer {MeReO(edt)}2 (1; where edt = 1,2-ethanedithiolate) catalyzes S atom transfer from thiiranes to triarylphosphines and triarylarsines. Despite the fact that phosphines are more nucleophilic than arsines, phosphines are less effective because they rapidly convert the dimer catalyst to the much less reactive catalyst [MeReO(edt)(PAr3)] (2). With AsAr3, which does not yield the monomer, the rate law is given by v = k[thiirane][1], independent of the arsine concentration. The values of k at 25.0 degrees C in CDCl3 are 5.58 +/- 0.08 L mol(-1) s(-1) for cyclohexene sulfide and ca. 2 L mol(-1) s(-1) for propylene sulfide. The activation parameters for cyclohexene sulfide are deltaH(double dagger) = 10.0 +/- 0.9 kcal mol(-1) and deltaS(double dagger) = -21 +/- 3 cal K(-1) mol(-1). Arsine enters the catalytic cycle after the rate-controlling release of alkene, undergoing a reaction with the Re(VII)(O)(S) intermediate that is so rapid in comparison that it cannot be studied directly. The use of a kinetic competition method provided relative rate constants and a Hammett reaction constant, rho = -1.0. Computations showed that there is little thermodynamic selectivity for arsine attack at O or S of the intermediate. There is, however, a large kinetic selectivity in favor of Ar3AsS formation: the calculated values of deltaH(double dagger) for attack of AsAr3 at Re=O vs Re=S in Re(VII)(O)(S) are 23.2 and 1.1 kcal mol(-1), respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
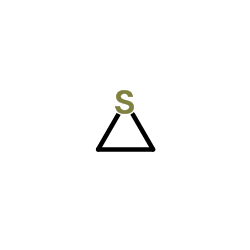 |
Thiirane
CAS:420-12-2 |
C2H4S |
|
Polymer complex of WR 2721. Synthesis and radioprotective ef...
2014-12-18 [Eur. J. Pharm. Sci. 65 , 9-14, (2014)] |
|
Reactivity of fullerene epoxide: preparation of fullerene-fu...
2008-04-04 [J. Org. Chem. 73(7) , 2518-26, (2008)] |
|
O-phenyl carbamate and phenyl urea thiiranes as selective ma...
2013-10-24 [J. Med. Chem. 56(20) , 8139-50, (2013)] |
|
Isolation of the key intermediates in the catalyst-free conv...
2009-04-07 [Org. Biomol. Chem. 7(7) , 1397-403, (2009)] |
|
The reactions of sulfur mustard with the active components o...
2005-08-31 [J. Hazard. Mater. 123(1-3) , 269-80, (2005)] |