| Structure | Name/CAS No. | Articles |
|---|---|---|
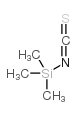 |
trimethylsilyl isothiocyanate
CAS:2290-65-5 |
|
 |
1,3-Dioxolane
CAS:646-06-0 |
|
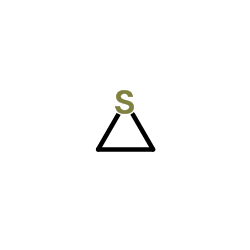 |
Thiirane
CAS:420-12-2 |