Inhibition of cytochrome P-450c-mediated benzo[a]pyrene hydroxylase and ethoxyresorufin O-deethylase by dihydrosafrole.
L R Kao, C F Wilkinson
Index: Xenobiotica 17(7) , 793-805, (1987)
Full Text: HTML
Abstract
1. Inhibitory activity of dihydrosafrole towards benzo[a]pyrene (BP) hydroxylase activity in hepatic microsomes from beta-naphthoflavone (BNF)-induced rats, and in reconstituted systems containing cytochrome P-450c, increased dramatically on preincubation of the inhibitor with NADPH; no inhibition occurred without preincubation. The level of BP hydroxylase inhibition was associated with the progressive formation of the 456 nm dihydrosafrole metabolite-cytochrome P-450c spectral complex during preincubation. 2. Inhibition of BP hydroxylase by dihydrosafrole in control microsomes, and inhibition of ethoxyresorufin O-deethylase (EROD) in microsomes (control or BNF-induced) and in reconstituted systems with cytochrome P-450c, did not require preincubation and apparently was not dependent on prior formation of the dihydrosafrole metabolite-cytochrome P-450 complex. 3. Kinetic studies established that, following preincubation with NADPH, dihydrosafrole was a noncompetitive inhibitor of both BP hydroxylase and EROD activities. In the absence of preincubation, dihydrosafrole was an effective competitive inhibitor of EROD in BNF-induced microsomes and in reconstituted systems with cytochrome P-450c. 4. Both ethoxyresorufin and benzo[a]pyrene inhibited the development of the type I optical difference spectrum of dihydrosafrole in reconstituted systems containing cytochrome P-450c. Inhibition by ethoxyresorufin was competitive while that caused by benzo[a]pyrene was noncompetitive in nature. 5. The type II ligand phenylimidazole was an effective noncompetitive inhibitor of EROD activity but failed to exert any inhibitory effect on cytochrome P-450c-mediated BP hydroxylase activity. Phenylimidazole inhibited formation of the dihydrosafrole type I optical difference spectrum non-competitively. 6. The results indicate that ethoxyresorufin and benzo[a]pyrene may occupy different binding sites on cytochrome P-450c and that dihydrosafrole binds primarily to the site utilized by ethoxyresorufin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
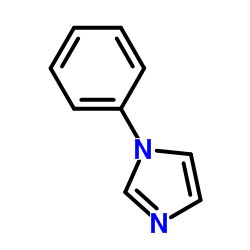 |
1-Phenylimidazole
CAS:7164-98-9 |
C9H8N2 |
|
Influence of inhibition of the metabolic activation on the m...
1988-11-01 [Mutat. Res. 202(1) , 251-67, (1988)] |
|
Calmodulin-dependent nitric-oxide synthase. Mechanism of inh...
1993-05-05 [J. Biol. Chem. 268(13) , 9425-9, (1993)] |
|
Three-dimensional pharmacophore hypotheses for the locust ne...
1999-07-01 [Bioorg. Med. Chem. 7(7) , 1437-43, (1999)] |
|
Acrolein genotoxicity in Drosophila melanogaster. III. Effec...
1994-05-01 [Mutat. Res. 321(3) , 119-26, (1994)] |
|
Chloroform-induced cytolethality in freshly isolated male B6...
1998-04-01 [Toxicol. Appl. Pharmacol. 149(2) , 217-25, (1998)] |