Synthesis and identification of the quaternary ammonium-linked glucuronide of 1-phenylimidazole in human liver microsomes and investigation of the human UDP-glucuronosyltransferases involved.
S C Vashishtha, E M Hawes, G McKay, D J McCann
Index: Drug Metab. Dispos. 28(9) , 1009-13, (2000)
Full Text: HTML
Abstract
1-Phenylimidazole was investigated as a potential model substrate with respect to formation of a quaternary ammonium-linked glucuronide (N(+)-glucuronide) at an aromatic type tertiary amine. A reference sample of the potential N(+)-glucuronide metabolite of 1-phenylimidazole was obtained by organic synthesis. The structural identity of the metabolite formed by incubation of 1-phenylimidazole with human liver microsomes was proven to be the N(+)-glucuronide by exhibiting the same HPLC retention time and electrospray ionization mass spectrum as the reference sample. The screening of 1-phenylimidazole against a panel of nine expressed human UDP-glucuronosyltransferases indicated the involvement of UGT1A3 and UGT1A4 in the formation of the N(+)-glucuronide metabolite.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
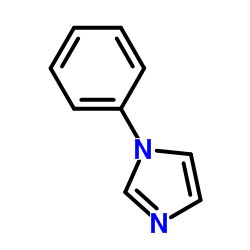 |
1-Phenylimidazole
CAS:7164-98-9 |
C9H8N2 |
|
Influence of inhibition of the metabolic activation on the m...
1988-11-01 [Mutat. Res. 202(1) , 251-67, (1988)] |
|
Calmodulin-dependent nitric-oxide synthase. Mechanism of inh...
1993-05-05 [J. Biol. Chem. 268(13) , 9425-9, (1993)] |
|
Three-dimensional pharmacophore hypotheses for the locust ne...
1999-07-01 [Bioorg. Med. Chem. 7(7) , 1437-43, (1999)] |
|
Acrolein genotoxicity in Drosophila melanogaster. III. Effec...
1994-05-01 [Mutat. Res. 321(3) , 119-26, (1994)] |
|
Chloroform-induced cytolethality in freshly isolated male B6...
1998-04-01 [Toxicol. Appl. Pharmacol. 149(2) , 217-25, (1998)] |