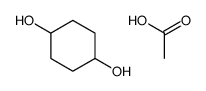
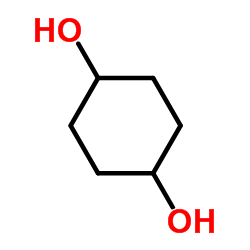
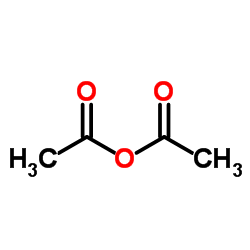
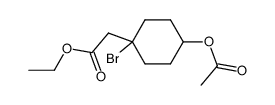
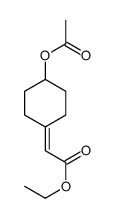
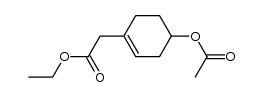
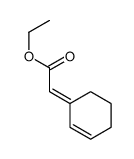
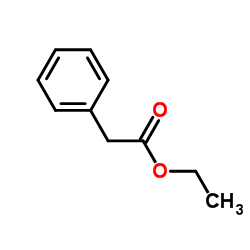
|
~88% |
|
~% |
|
~49% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |