7 alpha-hydroxylation of 26-hydroxycholesterol, 3 beta-hydroxy-5-cholestenoic acid and 3 beta-hydroxy-5-cholenoic acid by cytochrome P-450 in pig liver microsomes.
A Toll, J Shoda, M Axelson, J Sjövall, K Wikvall
Index: FEBS Lett. 296(1) , 73-6, (1992)
Full Text: HTML
Abstract
Pig liver microsomes were found to catalyze the 7 alpha-hydroxylation of several potential bile acid precursors besides cholesterol. 26-Hydroxycholesterol, 3 beta-hydroxy-5-cholestenoic acid and 3 beta-hydroxy-5-cholenoic acid were all efficiently converted into the 7 alpha-hydroxylated products. Two cytochrome P-450 fractions showing 7 alpha-hydroxylase activity could be isolated. One fraction catalyzed 7 alpha-hydroxylation of 26-hydroxycholesterol, 3 beta-hydroxy-5-cholestenoic acid and 3 beta-hydroxy-5-cholenoic acid but was inactive towards cholesterol. The other fraction catalyzed 7 alpha-hydroxylation of cholesterol in addition to the other substrates. 26-Hydroxycholesterol in equimolar concentration did not inhibit the cholesterol 7 alpha-hydroxylase activity of this fraction. It is concluded that liver microsomes contain a cytochrome P-450 catalyzing 7 alpha-hydroxylation of 26-hydroxycholesterol, 3 beta-hydroxy-5-cholestenoic acid and 3 beta-hydroxy-5-cholenoic acid. The results indicate that this cytochrome P-450 is different from that catalyzing 7 alpha-hydroxylation of cholesterol.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
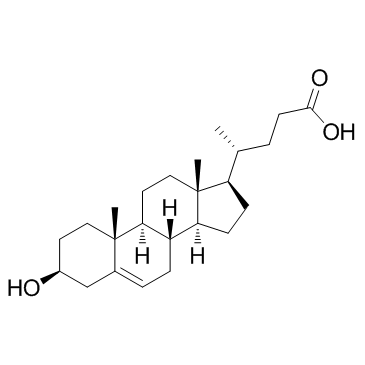 |
3b-Hydroxy-5-cholenoic acid
CAS:5255-17-4 |
C24H38O3 |
|
Hepatic bile salt sulfotransferases in the rat: sulfation of...
1991-02-01 [J. Pediatr. Gastroenterol. Nutr. 12(2) , 260-8, (1991)] |
|
Determination of 3 beta-hydroxy-5-cholen-24-oic acid and its...
1984-07-01 [Steroids 44(1) , 47-55, (1984)] |
|
Unusual serum bile acid pattern in children with the syndrom...
1985-02-15 [Clin. Chim. Acta 145(3) , 289-96, (1985)] |
|
Purification and properties of a novel sulfatase from Pseudo...
1998-09-01 [Biosci. Biotechnol. Biochem. 62(9) , 1739-44, (1998)] |
|
Preparation and antigenic property of methyl 3 beta-hydroxy-...
1983-02-01 [Steroids 41(2) , 155-64, (1983)] |