Multiplicity of in vitro glucuronidation of 2-hydroxyestriol.
T Ohkubo, A Takahashi, T Nambara
Index: J. Steroid Biochem. 36(5) , 501-3, (1990)
Full Text: HTML
Abstract
In vitro glucuronidation of 2-hydroxyestriol has been investigated by means of HPLC with dual-electrode coulometric detection. When incubated with rat or dog liver microsomal preparation in the presence of UDPGA, 2-hydroxyestriol was transformed into the 2-glucuronide together with a small amount of 16- and/or 17-glucuronides. In contrast, incubation of 2-hydroxyestriol with guinea-pig liver microsomal preparation yielded the 3-glucuronide and a trace amount of the 2-glucuronide, but no ring D glucuronides. Upon pretreatment with 3-methylcholanthrene male rat liver exhibited a marked increase in both 2- and 3-glucuronidation activities, whereas female rat liver showed an elevation only in 2-glucuronidation. On the other hand, in male and female rats pretreatment with phenobarbital caused a relatively small increase in the glucuronidation activity of the liver. In the male guinea-pig, glucuronidation was not affected by pretreatment with either of the two compounds. The present result demonstrates the multiplicity of hepatic 2-hydroxyestriol UDP-glucuronyl-transferase in the rat, guinea-pig and dog.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
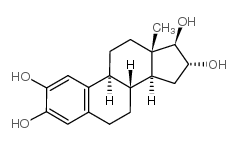 |
2-Hydroxyestriol
CAS:1232-80-0 |
C18H24O4 |
|
Simultaneous perfusion of [4-14C]oestriol and [6,9-3H2]oestr...
1982-06-01 [Acta Endocrinol. 100(2) , 274-8, (1982)] |
|
Comparison of metabolic ratios of urinary estrogens between ...
2013-01-01 [BMC Clin. Pathol. 13 , 25, (2014)] |
|
Urinary hydroxyestrogens and breast cancer risk among postme...
2005-09-01 [Cancer Epidemiol. Biomarkers Prev. 14(9) , 2137-42, (2005)] |
|
Novel and potent biological antioxidants on membrane phospho...
1987-02-13 [Biochem. Biophys. Res. Commun. 142(3) , 919-24, (1987)] |
|
Synthesis of 2-hydroxyestriol monoglucuronides and monosulfa...
1990-03-01 [Steroids 55(3) , 128-32, (1990)] |