| Structure | Name/CAS No. | Articles |
|---|---|---|
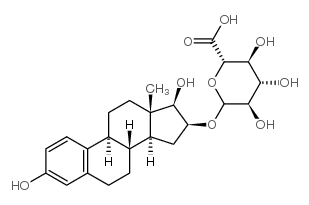 |
Estriol 16α-(β-D-glucuronide)
CAS:1852-50-2 |
|
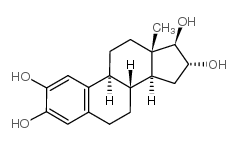 |
2-Hydroxyestriol
CAS:1232-80-0 |
|
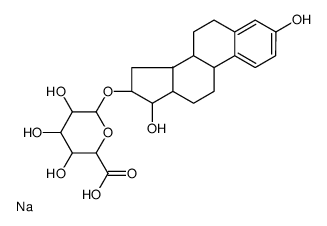 |
Estriol 16α-(β-D-glucuronide) sodium salt
CAS:1852-44-4 |