Synthesis of 2-hydroxyestriol monoglucuronides and monosulfates.
T Ohkubo, T Wakasawa, T Nambara
Index: Steroids 55(3) , 128-32, (1990)
Full Text: HTML
Abstract
The ring A monoglucuronides and monosulfates of 2-hydroxyestriol were synthesized from 2-hydroxyestriol 16,17-diacetate by means of the Koenigs-Knorr reaction with methyl alpha-acetobromoglucuronate and sulfation with sulfur trioxide-pyridine complex, respectively. The conjugated positions of these compounds were definitely established by conversion to 2-hydroxyestriol monomethyl ethers by methylation, then enzymatic hydrolysis. The ring D monoglucuronides and monosulfates of 2-hydroxyestriol were also prepared from 2-hydroxyestriol 2,3-dibenzyl ether by glucuronidation and sulfation in a similar fashion followed by debenzylation, respectively. The positions of conjugation were established on the basis of their 1H-nuclear magnetic resonance spectral data.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
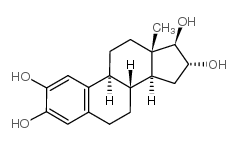 |
2-Hydroxyestriol
CAS:1232-80-0 |
C18H24O4 |
|
Simultaneous perfusion of [4-14C]oestriol and [6,9-3H2]oestr...
1982-06-01 [Acta Endocrinol. 100(2) , 274-8, (1982)] |
|
Comparison of metabolic ratios of urinary estrogens between ...
2013-01-01 [BMC Clin. Pathol. 13 , 25, (2014)] |
|
Urinary hydroxyestrogens and breast cancer risk among postme...
2005-09-01 [Cancer Epidemiol. Biomarkers Prev. 14(9) , 2137-42, (2005)] |
|
Novel and potent biological antioxidants on membrane phospho...
1987-02-13 [Biochem. Biophys. Res. Commun. 142(3) , 919-24, (1987)] |
|
Effect of catechol estrogens and estriol on the induction of...
1980-06-01 [J. Steroid Biochem. 13(6) , 681-3, (1980)] |