VUV photon-induced ionization/dissociation of antipyrine and propyphenazone: mass spectrometric and theoretical insights.
Liulin Deng, Lidong Zhang, Huijun Guo, Liangyuan Jia, Yang Pan, Hao Yin, Fei Qi
Index: J. Mass Spectrom. 45(7) , 734-9, (2010)
Full Text: HTML
Abstract
Two analgesic and anti-inflammatory drugs, antipyrine and propyphenazone, were investigated with infrared laser desorption/tunable synchrotron vacuum ultraviolet (VUV) photoionization mass spectrometry (IR LD/VUV PIMS) and theoretical calculations. Mass spectra of the two drugs were measured at various photon energies. Fragment ions were gradually produced as photon energy increases. The structural assignment of the dominant fragment ions was supported by the results from a commercial electron impact time-of-flight mass spectrometer (EI-TOF MS). Primary fragmentation pathways were established from experimental observations combining with theoretical calculations. Methyl radical elimination is a common fragmentation pathway for two analytes. However, for propyphenazone cation, isopropyl group elimination to form antipyrine cation is another competitive pathway.2010 John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
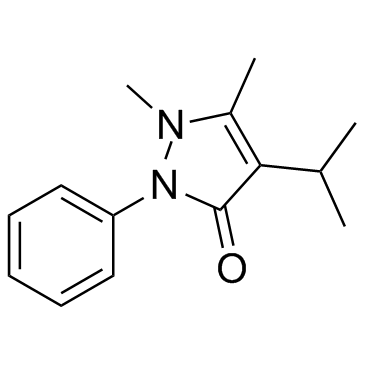 |
propyphenazone
CAS:479-92-5 |
C14H18N2O |
|
Development of a multicommuted flow-through optosensor for t...
2007-01-17 [J. Pharm. Biomed. Anal. 43(2) , 515-21, (2007)] |
|
Green chromatography separation of analytes of greatly diffe...
2013-07-01 [Anal. Bioanal. Chem 405(18) , 6105-15, (2013)] |
|
Removal of selected pharmaceuticals by chlorination, coagula...
2008-01-01 [Water Sci. Technol. 58(5) , 1129-35, (2008)] |
|
Quantification of 4-methylaminoantipyrine, the active metabo...
2009-05-01 [Bioanalysis 1(2) , 293-8, (2009)] |
|
Safety of the new selective cyclooxygenase type 2 inhibitors...
2004-10-01 [Ann. Allergy Asthma Immunol. 93(4) , 360-4, (2004)] |