Green chromatography separation of analytes of greatly differing properties using a polyethylene glycol stationary phase and a low-toxic water-based mobile phase.
Dalibor Šatínský, Ivana Brabcová, Alena Maroušková, Petr Chocholouš, Petr Solich
Index: Anal. Bioanal. Chem 405(18) , 6105-15, (2013)
Full Text: HTML
Abstract
A simple, rapid, and environmentally friendly HPLC method was developed and validated for the separation of four compounds (4-aminophenol, caffeine, paracetamol, and propyphenazone) with different chemical properties. A "green" mobile phase, employing water as the major eluent, was proposed and applied to the separation of analytes with different polarity on polyethylene glycol (PEG) stationary phase. The chromatography separation of all compounds and internal standard benzoic acid was performed using isocratic elution with a low-toxicity mobile phase consisting of 0.04% (v/v) triethylamine and water. HPLC separation was carried out using a PEG reversed-phase stationary phase Supelco Discovery HS PEG column (15 × 4 mm; particle size 3 μm) at a temperature of 30 °C and flow rate at 1.0 mL min(-1). The UV detector was set at 210 nm. In this study, a PEG stationary phase was shown to be suitable for the efficient isocratic separation of compounds that differ widely in hydrophobicity and acid-base properties, particularly 4-aminophenol (log P, 0.30), caffeine (log P, -0.25), and propyphenazone (log P, 2.27). A polar PEG stationary phase provided specific selectivity which allowed traditional chromatographic problems related to the separation of analytes with different polarities to be solved. The retention properties of the group of structurally similar substances (aromatic amines, phenolic compounds, and xanthine derivatives) were tested with different mobile phases. The proposed green chromatography method was successfully applied to the analysis of active substances and one degradation impurity (4-aminophenol) in commercial preparation. Under the optimum chromatographic conditions, standard calibration was carried out with good linearity correlation coefficients for all compounds in the range (0.99914-0.99997, n = 6) between the peak areas and concentration of compounds. Recovery of the sample preparation was in the range 100 ± 5% for all compounds. The intraday method precision was determined as RSD, and the values were lower than 1.00%.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
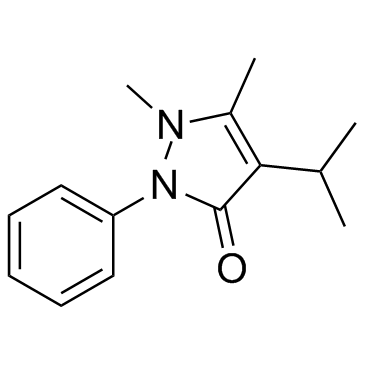 |
propyphenazone
CAS:479-92-5 |
C14H18N2O |
|
Development of a multicommuted flow-through optosensor for t...
2007-01-17 [J. Pharm. Biomed. Anal. 43(2) , 515-21, (2007)] |
|
VUV photon-induced ionization/dissociation of antipyrine and...
2010-07-01 [J. Mass Spectrom. 45(7) , 734-9, (2010)] |
|
Removal of selected pharmaceuticals by chlorination, coagula...
2008-01-01 [Water Sci. Technol. 58(5) , 1129-35, (2008)] |
|
Quantification of 4-methylaminoantipyrine, the active metabo...
2009-05-01 [Bioanalysis 1(2) , 293-8, (2009)] |
|
Safety of the new selective cyclooxygenase type 2 inhibitors...
2004-10-01 [Ann. Allergy Asthma Immunol. 93(4) , 360-4, (2004)] |