The solution structure of the Josephin domain of ataxin-3: structural determinants for molecular recognition.
Giuseppe Nicastro, Rajesh P Menon, Laura Masino, Philip P Knowles, Neil Q McDonald, Annalisa Pastore
Index: Proc. Natl. Acad. Sci. U. S. A. 102(30) , 10493-8, (2005)
Full Text: HTML
Abstract
The Josephin domain plays an important role in the cellular functions of ataxin-3, the protein responsible for the neurodegenerative Machado-Joseph disease. We have determined the solution structure of Josephin and shown that it belongs to the family of papain-like cysteine proteases, sharing the highest degree of structural similarity with bacterial staphopain. A currently unique structural feature of Josephin is a flexible helical hairpin formed by a 32-residue insertion, which could determine substrate specificity. By using the Josephin structure and the availability of NMR chemical shift assignments, we have mapped the enzyme active site by using the typical cysteine protease inhibitors, transepoxysuccinyl-L-eucylamido-4-guanidino-butane (E-64) and [L-3-trans-(propylcarbamyl)oxirane-2-carbonyl]-L-isoleucyl-L-proline (CA-074). We also demonstrate that the specific interaction of Josephin with the ubiquitin-like domain of the ubiquitin- and proteasome-binding factor HHR23B involves complementary exposed hydrophobic surfaces. The structural similarity with other deubiquitinating enzymes suggests a model for the proteolytic enzymatic activity of ataxin-3.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
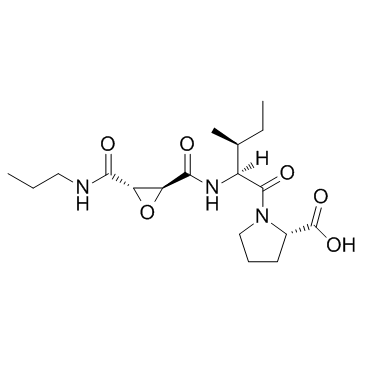 |
CA 074 TFA
CAS:134448-10-5 |
C18H29N3O6 |
|
Human cystatin C: a new biomarker of idiopathic pulmonary fi...
2014-06-01 [Proteomics. Clin. Appl. 8(5-6) , 447-53, (2014)] |
|
Redox-based inactivation of cysteine cathepsins by compounds...
2011-01-01 [PLoS ONE 6(11) , e27197, (2011)] |
|
Structural basis for development of cathepsin B-specific non...
2002-06-03 [Biochim. Biophys. Acta 1597(2) , 244-51, (2002)] |
|
Primate neurons show different vulnerability to transient is...
2002-09-01 [Acta Neuropathol. 104(3) , 267-72, (2002)] |
|
Role of the mitochondria in immune-mediated apoptotic death ...
2011-01-01 [PLoS ONE 6 , e20617, (2011)] |