| Structure | Name/CAS No. | Articles |
|---|---|---|
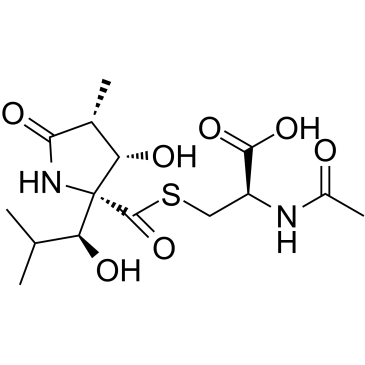 |
Lactacystin
CAS:133343-34-7 |
|
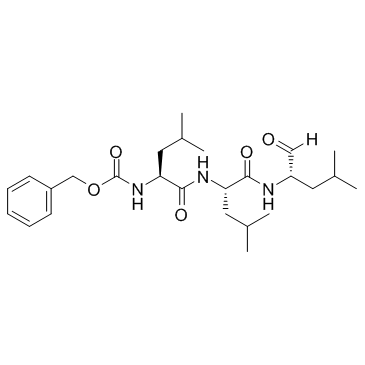 |
MG-132
CAS:133407-82-6 |
|
 |
Ubiquitin (from bovine erythrocytes)
CAS:79586-22-4 |
|
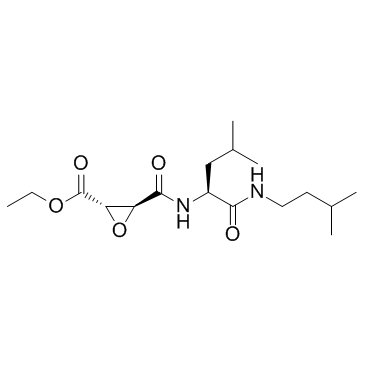 |
E-64d
CAS:88321-09-9 |