| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
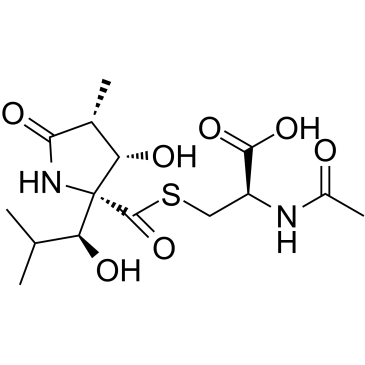 |
乳胞素
CAS:133343-34-7 |
|
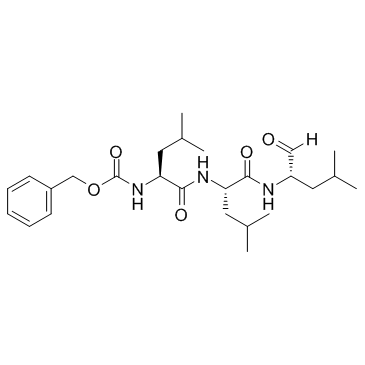 |
蛋白酶体抑制剂
CAS:133407-82-6 |
|
 |
泛素
CAS:79586-22-4 |
|
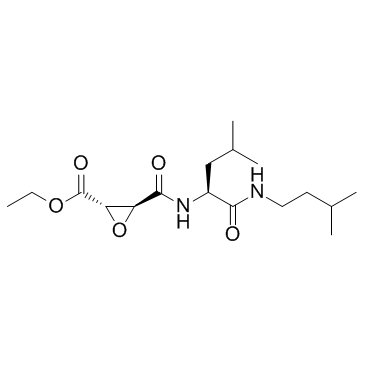 |
阿洛司他丁
CAS:88321-09-9 |