The structure of (S)-(-)-4-(2-benzimidazolyl)-4-hydroxybutyric acid formed during mammalian metabolism of the fungicide 2-(2-furyl)benzimidazole.
Adrian Frank, Rolf Norrestam
Index: Basic Clin Pharmacol Toxicol. 96(4) , 325-30, (2005)
Full Text: HTML
Abstract
During metabolism of the fungicide 2-(2-furyl)benzimidazole (FB) in mammals, an optically active compound, metabolite A, was isolated. This compound, a principal metabolite in the biological degradation of FB, was isolated from the urine of horse and dog. When studied by IR, NMR, MS, CD and electrophoretic techniques, the metabolite proved to be the zwitterionic form of (S)-(-)-4-(2-benzimidazolyl)-4-hydroxybutyric acid. The synthesized optical active compound was in every respect identical with metabolite A. The suggested structure has been completely verified by the current investigation of its crystal structure, based on single crystal diffraction data collected at the synchrotron MAX II in Lund, Sweden. In an earlier determination, a related compound, 3-benzimidazolylpropionic acid (Ercan et al. 1996) was suggested to be non-zwitterionic. The present studies have shown that the latter compound too is zwitterionic and that the conclusions presented were apparently based on some erroneously proposed hydrogen positions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
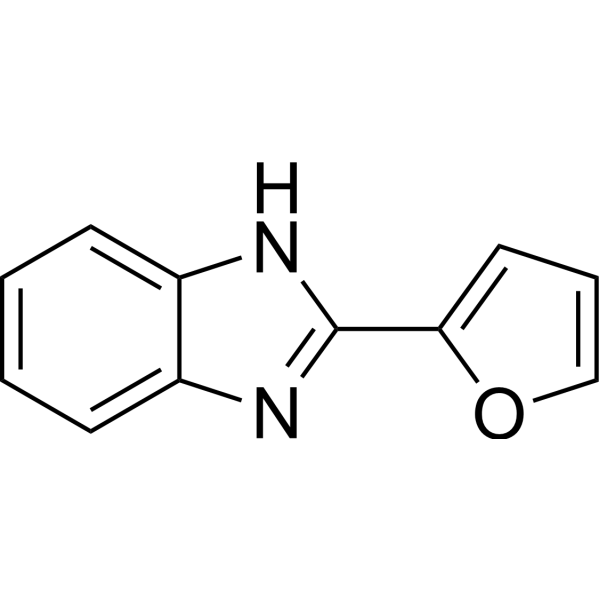 |
Fuberidazole
CAS:3878-19-1 |
C11H8N2O |
|
Subtype-selectivity of metal-dependent methionine aminopepti...
2010-07-15 [Bioorg. Med. Chem. Lett. 20 , 4038-44, (2010)] |
|
Metal-mediated inhibition of Escherichia coli methionine ami...
2006-01-26 [J. Med. Chem. 49 , 511-22, (2006)] |
|
Quantitative Fusarium spp. and Microdochium spp. PCR assays ...
2007-06-01 [J. Appl. Microbiol. 102(6) , 1645-53, (2007)] |
|
Possible bitertanol and fuberidazole poisoning in young phea...
2001-07-28 [Vet. Rec. 149(4) , 118-20, (2001)] |
|
Determination of benzimidazolic fungicides in fruits and veg...
2009-09-21 [Anal. Chim. Acta 650(2) , 207-13, (2009)] |