Dearomatizing benzene ring reductases.
Matthias Boll
Index: J. Mol. Microbiol. Biotechnol. 10(2-4) , 132-42, (2005)
Full Text: HTML
Abstract
The high resonance energy of the benzene ring is responsible for the relative resistance of aromatic compounds to biodegradation. Nevertheless, bacteria from nearly all physiological groups have been isolated which utilize aromatic growth substrates as the sole source of cell carbon and energy. The enzymatic dearomatization of the benzene nucleus by microorganisms is accomplished in two different manners. In aerobic bacteria the aromatic ring is dearomatized by oxidation, catalyzed by oxygenases. In contrast, anaerobic bacteria attack the aromatic ring by reductive steps. Key intermediates in the anaerobic aromatic metabolism are benzoyl-CoA and compounds with at least two meta-positioned hydroxyl groups (resorcinol, phloroglucinol and hydroxyhydroquinone). In facultative anaerobes, the reductive dearomatization of the key intermediate benzoyl-CoA requires a stoichiometric coupling to ATP hydrolysis, whereas reduction of the other intermediates is readily achieved with suitable electron donors. Obligately anaerobic bacteria appear to use a totally different enzymology for the reductive dearomatization of benzoyl-CoA including selenocysteine- and molybdenum- containing enzymes.Copyright 2005 S. Karger AG, Basel.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
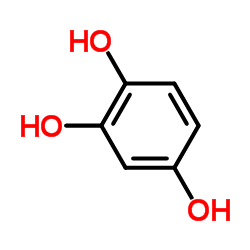 |
1,2,4-Trihydroxybenzene
CAS:533-73-3 |
C6H6O3 |
|
A convenient screening method to differentiate phenolic skin...
2015-10-01 [J. Dermatol. Sci. 80 , 18-24, (2015)] |
|
Degradation of sulfonamide antibiotics by Microbacterium sp....
2015-12-25 [New Biotechnology 32 , 710-5, (2015)] |
|
The TetR-type transcriptional repressor RolR from Corynebact...
2012-09-01 [Appl. Environ. Microbiol. 78(17) , 6009-16, (2012)] |
|
Phenolic metabolites of benzene induced caspase-dependent cy...
2014-12-01 [Environ. Toxicol. 29(12) , 1437-51, (2014)] |
|
Solvent effect on pathways and mechanisms for D-fructose con...
2013-03-14 [J. Phys. Chem. A 117(10) , 2102-13, (2013)] |