Nucleophilic additions of trimethylsilyl cyanide to cyclic oxocarbenium ions: evidence for the loss of stereoselectivity at the limits of diffusion control.
Siddhartha R Shenoy, Deborah M Smith, K A Woerpel
Index: J. Am. Chem. Soc. 128(26) , 8671-7, (2006)
Full Text: HTML
Abstract
The limitations of stereoelectronic models in assessing the stereoselective nucleophilic substitution reactions of cyclic oxocarbenium ions at high reaction rates are discussed. Evidence is provided suggesting that the diastereoselectivity of nucleophilic substitution reactions is attenuated at the limits of diffusion control. The low diastereoselectivities observed in the reactions of trimethylsilyl cyanide with five- and six-membered ring oxocarbenium ions are attributed to the high reactivity of the nucleophile and its reactions with these electrophiles at diffusion control rates.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
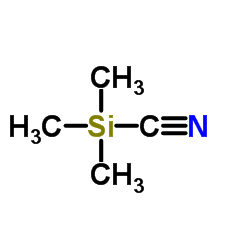 |
Trimethylsilyl cyanide
CAS:7677-24-9 |
C4H9NSi |
|
Asymmetric activation of tropos 2,2'-biphenol with cinchonin...
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46(44) , 8468-70, (2007)] |
|
A chemoselective Ugi-type reaction in water using TMSCN as a...
2012-12-14 [Org. Biomol. Chem. 10(46) , 9271-7, (2012)] |
|
Sc₂(pydc)₂ unit based 1D, 2D and 3D metal-organic frameworks...
2015-01-28 [Dalton Trans. 44(4) , 1942-7, (2014)] |
|
Facile and efficient enantioselective Strecker reaction of k...
2009-06-08 [Chemistry 15(24) , 6008-14, (2009)] |
|
Synthesis of homo-N-nucleoside with 1,2,4-triazole-3-carboxa...
2005-01-01 [Nucleosides Nucleotides Nucleic Acids 24(5-7) , 979-81, (2005)] |