Bioorganic & Medicinal Chemistry Letters
2010-02-01
A study of the effects of substituents on the selectivity of the binding of N-arylaminomethylene malonate inhibitors to DHODH.
Deborah Cowen, Paul Bedingfield, Glenn A McConkey, Colin W G Fishwick, A Peter Johnson
Index: Bioorg. Med. Chem. Lett. 20(3) , 1284-7, (2010)
Full Text: HTML
Abstract
A series of mono- and di-substituted N-arylaminomethylene malonates have been used to probe the potential of utilizing additional H-bonding contacts in the ubiquinone binding channel, for selective inhibition between either human or Plasmodium DHODH. Altered 'head' group functionalities have been utilized in order to probe the role of specific functionalities within the inhibitors in terms of enzyme affinity and selectivity.Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
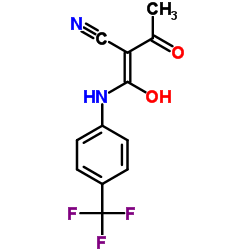 |
teriflunomide
CAS:108605-62-5 |
C12H9F3N2O2 |
Related Articles:
More...
|
Hepatic cytochrome P450s attenuate the cytotoxicity induced ...
2011-08-01 [Toxicol. Sci. 122(2) , 579-86, (2011)] |
|
Respiratory syncytial virus inhibits lung epithelial Na+ cha...
2009-03-13 [J. Biol. Chem. 284(11) , 7294-306, (2009)] |
|
Induction of EMT-like phenotypes by an active metabolite of ...
2010-12-01 [Cell Death Differ. 17(12) , 1882-95, (2010)] |
|
Postinfection A77-1726 treatment improves cardiopulmonary fu...
2012-10-01 [Am. J. Respir. Cell. Mol. Biol. 47(4) , 543-51, (2012)] |
|
Inhibiting effects of Leflunomide metabolite on overexpressi...
2011-11-16 [Eur. J. Pharmacol. 670(1) , 304-10, (2011)] |