Search for influence of spatial properties on affinity at α1-adrenoceptor subtypes for phenylpiperazine derivatives of phenytoin.
Jadwiga Handzlik, Heinz H Pertz, Tilo Görnemann, Sven Jähnichen, Katarzyna Kieć-Kononowicz
Index: Bioorg. Med. Chem. Lett. 20(20) , 6152-6, (2010)
Full Text: HTML
Abstract
A series of phenylpiperazine derivatives of phenytoin was evaluated for their affinity at α(1)-adrenoceptor subtypes in functional bioassays (rat tail artery: α(1A) and/or α(1B); guinea pig spleen: α(1B); rat aorta: α(1D)). The most potent compounds at α(1A)-, α(1B)- and α(1D)-adrenoceptors, 11, 18 and 8, showed affinities in the submicromolar range. The role of a hydrogen bond donor group for affinity and selectivity at α(1B)-adrenoceptors, postulated by Bremner's pharmacophore model, was confirmed by functional and molecular modelling studies.Copyright © 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
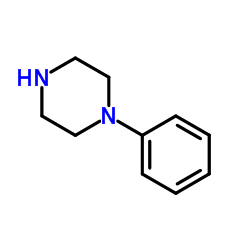 |
1-Phenylpiperazine
CAS:92-54-6 |
C10H14N2 |
|
Synthesis and evaluation of a set of 4-phenylpiperidines and...
2010-03-25 [J. Med. Chem. 53(6) , 2510-20, (2010)] |
|
Dopamine D2, D3, and D4 selective phenylpiperazines as molec...
2009-08-13 [J. Med. Chem. 52(15) , 4923-35, (2009)] |
|
Analysis of phenylpiperazine-like stimulants in human hair a...
2010-10-01 [J. Chromatogr. A. 1217(40) , 6274-80, (2010)] |
|
Sleep deprivation differentially affects dopamine receptor s...
2011-07-13 [Neuroreport 22(10) , 489-93, (2011)] |
|
DNA-binding, spectroscopic and antimicrobial studies of pall...
2012-10-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 96 , 586-93, (2012)] |