| Structure | Name/CAS No. | Articles |
|---|---|---|
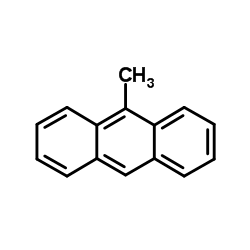 |
9-Methylanthracene
CAS:779-02-2 |
|
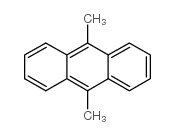 |
9,10-Dimethylanthracene
CAS:781-43-1 |
|
 |
9-Anthrylmethanol
CAS:1468-95-7 |