| Structure | Name/CAS No. | Articles |
|---|---|---|
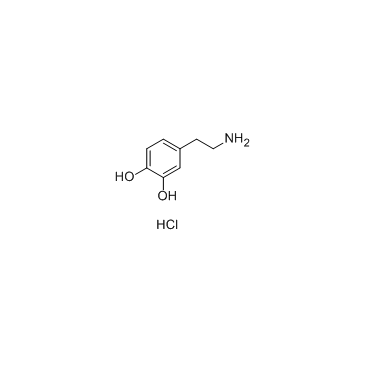 |
Dopamine hydrochloride
CAS:62-31-7 |
|
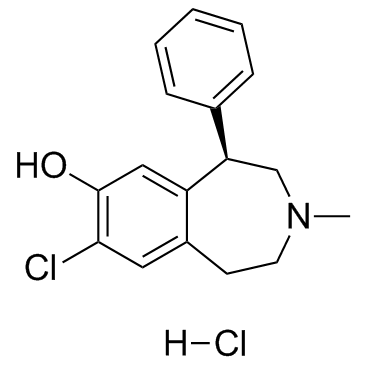 |
SCH 23390 hydrochloride
CAS:125941-87-9 |
|
 |
R(+)-SKF-38393A
CAS:81702-42-3 |