| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
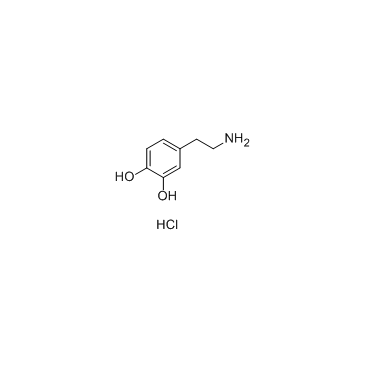 |
盐酸多巴胺
CAS:62-31-7 |
|
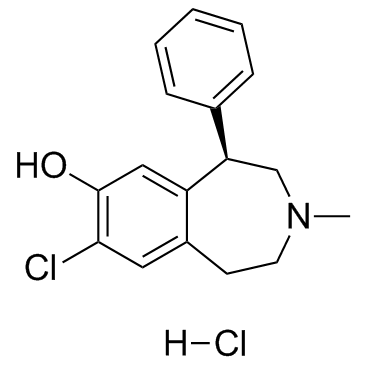 |
SCH 23390盐酸盐
CAS:125941-87-9 |
|
 |
(R)-(+)-SKF-38393 盐酸盐
CAS:81702-42-3 |