Site-specific binding of quinones to proteins through thiol addition and addition-elimination reactions.
Wen-Wu Li, Jürgen Heinze, Wolfgang Haehnel
Index: J. Am. Chem. Soc. 127(17) , 6140-1, (2005)
Full Text: HTML
Abstract
Ubiquinone-0, menaquinone-0, and 2,3,5-trimethyl-1,4-benzoquinone were site-specifically bound to free cysteine of proteins (yeast iso-1 cytochrome c as a model protein) through thioether bond formation. Model thioether quinone conjugates showed unexpected reactivity to cysteine of proteins as their parent quinones by thiol addition-elimination reaction. Cyclic voltammetry studies of the model compounds showed only minor differences in their redox potentials as compared to their parent quinones. Thioether ligation provides a general, simple, and fast method to construct model quinone protein systems. In addition, these studies also contribute to the understanding of biological activities, toxicity, and anti-cancer mechanism of quinones and thioether quinone adducts.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
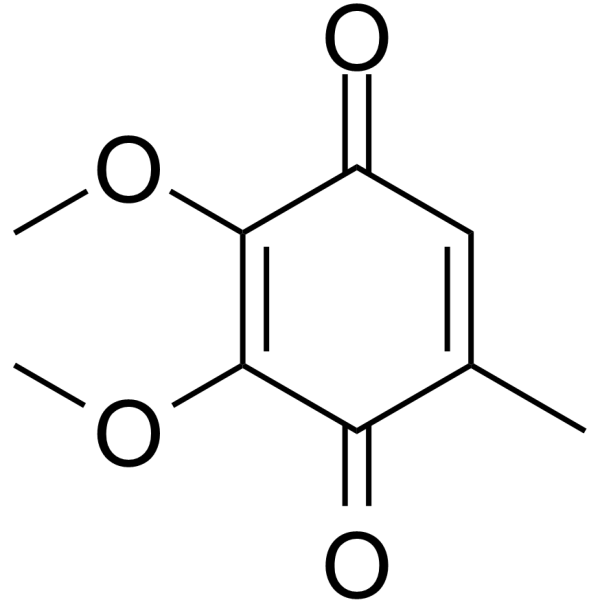 |
Ubiquinone Q0
CAS:605-94-7 |
C9H10O4 |
|
Cofactor Specificity of the Bifunctional Alcohol and Aldehyd...
2015-08-01 [J. Bacteriol. 197 , 2610-9, (2015)] |
|
Prerequisites for ubiquinone analogs to prevent mitochondria...
2012-02-01 [J. Bioenerg. Biomembr. 44(1) , 207-12, (2012)] |
|
Ubiquinone analogs: a mitochondrial permeability transition ...
2010-01-01 [PLoS ONE 5 , e11792, (2010)] |
|
Immunochemical identification of coenzyme Q0-dihydrolipoamid...
2004-06-25 [J. Biol. Chem. 279 , 27278-27285, (2004)] |
|
Photolabile ubiquinone analogues for identification and char...
2010-01-01 [Bioorg. Med. Chem. 18 , 3457-66, (2010)] |