Crosslinking of phenylalanyl-tRNA to the ribosomal A site via a photoaffinity probe attached to the 4-thiouridine residue is exclusively to ribosomal protein S19.
F L Lin, L Kahan, J Ofengand
Index: J. Mol. Biol. 172(1) , 77-86, (1984)
Full Text: HTML
Abstract
Phe-tRNA of Escherichia coli, specifically derivatized at the S4U8 position with the 9 A long p-azidophenacyl photoaffinity probe, can be crosslinked to 30 S ribosomal protein when the tRNA is placed at the ribosomal A site. This protein has now been identified by immunological methods. The protein-[3H]Phe-tRNA covalent complex, obtained by extraction with 6 M-urea, was reacted separately with each of the 21 purified antisera to 30 S ribosomal proteins. The double antibody technique was used. Anti-S19 was the only antiserum able to precipitate the radioactivity, and 66 to 81% of the added radioactivity could be precipitated. The same result was obtained with three different ribosome preparations, at low as well as high crosslinking yield, with dipeptidyl-tRNA in the A site as well as aminoacyl-tRNA, and when binding and crosslinking were performed at 20 mM-Mg2+ instead of at 5 mM. Therefore, when aminoacyl-tRNA or peptidyl-tRNA is in the ribosomal A site, position 8, which is always uridine or 4-thiouridine, must be within 9 A of protein S19.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
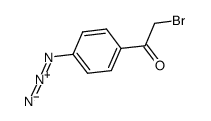 |
4-azidophenacyl bromide
CAS:57018-46-9 |
C8H6BrN3O |
|
Direct identification of on-bead peptides using surface-enha...
2015-01-01 [Sci. Rep. 5 , 10144, (2015)] |
|
The σ70 region 1.2 regulates promoter escape by unwinding DN...
2013-04-01 [Nucleic Acids Res. 41(8) , 4565-72, (2013)] |
|
4-Bromophenacyl bromide specifically inhibits rhoptry secret...
2009-01-01 [PLoS ONE 4(12) , e8143, (2009)] |
|
Photoaffinity reagents for use with pepsin and other carboxy...
1983-03-16 [Biochem. Biophys. Res. Commun. 111(2) , 630-5, (1983)] |
|
Association of thioredoxin with the inner membrane and adhes...
1987-06-01 [J. Bacteriol. 169(6) , 2659-66, (1987)] |