| Structure | Name/CAS No. | Articles |
|---|---|---|
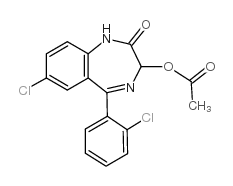 |
7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2-oxo-2H-1,4-benzodiazepin-3-yl acetate
CAS:2848-96-6 |
I Fitos, J Visy, M Simonyi, J Hermansson
Index: Chirality 11(2) , 115-20, (1999)
Full Text: HTML
The effect of ibuprofen enantiomers on the stereoselective binding of 3-acyloxy-1,4-benzodiazepines to human serum albumin (HSA) was studied using both native and Sepharose-immobilized protein. (S)-Lorazepam acetate exhibited considerably enhanced binding, especially in the presence of (+)-(S)-ibuprofen. The phenomenon is an indication of cooperative allosteric interaction between different binding sites during multiple cobinding of two ligands.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2-oxo-2H-1,4-benzodiazepin-3-yl acetate
CAS:2848-96-6 |
C17H12Cl2N2O3 |
|
Stereoselective binding of 3-acetoxy-, and 3-hydroxy-1,4-ben...
1986-01-15 [Biochem. Pharmacol. 35(2) , 263-9, (1986)] |
|
Stereoselective kinetics of warfarin binding to human serum ...
2002-05-15 [Chirality 14(5) , 442-8, (2002)] |
|
Enantioselective hydrolysis of lorazepam 3-acetate by estera...
1991-01-01 [Drug Metab. Dispos. 19(3) , 609-13, (1991)] |
|
Recurrent complex partial status epilepticus associated with...
2002-03-01 [Acta Neurol. Belg. 102(1) , 19-20, (2002)] |
|
Application of ultrafiltration and CD spectroscopy for study...
1982-12-15 [Biochem. Biophys. Res. Commun. 109(3) , 851-7, (1982)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved