Stereoselective binding of 3-acetoxy-, and 3-hydroxy-1,4-benzodiazepine-2-ones to human serum albumin. Selective allosteric interaction with warfarin enantiomers.
I Fitos, Z Tegyey, M Simonyi, I Sjöholm, T Larsson, C Lagercrantz
Index: Biochem. Pharmacol. 35(2) , 263-9, (1986)
Full Text: HTML
Abstract
Stereoselective binding of oxazepam, lorazepam, temazepam and methyl lorazepam as well as of their acetates to human serum albumin was investigated by different techniques. The 2'-chlorine and the N(1)-methyl substitution exert opposite effects on the antipodes. Enantiomers of oxazepam acetate (OAc) and lorazepam acetate (LAc) displace diazepam. Allosteric interactions with warfarin were manifested by either mutually increased or decreased binding depending on the structure of benzodiazepine and on the configuration of both benzodiazepine and warfarin. The most remarkable effect could be observed in the simultaneous binding of (S)-lorazepam acetate and (S)-warfarin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
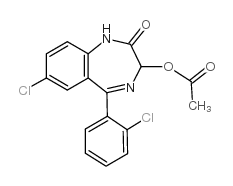 |
7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2-oxo-2H-1,4-benzodiazepin-3-yl acetate
CAS:2848-96-6 |
C17H12Cl2N2O3 |
|
Stereoselective kinetics of warfarin binding to human serum ...
2002-05-15 [Chirality 14(5) , 442-8, (2002)] |
|
Stereoselective allosteric binding interaction on human seru...
1999-01-01 [Chirality 11(2) , 115-20, (1999)] |
|
Enantioselective hydrolysis of lorazepam 3-acetate by estera...
1991-01-01 [Drug Metab. Dispos. 19(3) , 609-13, (1991)] |
|
Recurrent complex partial status epilepticus associated with...
2002-03-01 [Acta Neurol. Belg. 102(1) , 19-20, (2002)] |
|
Application of ultrafiltration and CD spectroscopy for study...
1982-12-15 [Biochem. Biophys. Res. Commun. 109(3) , 851-7, (1982)] |