Studies on the relationships between 7 alpha-hydroxylation and the ability of 25- and 27-hydroxycholesterol to suppress the activity of HMG-CoA reductase.
J Zhang, A Dricu, J Sjövall
Index: Biochim. Biophys. Acta 1344(3) , 241-9, (1997)
Full Text: HTML
Abstract
The metabolism of 25-hydroxycholesterol in different cell types was studied and the role of 7 alpha-hydroxylation for the effect of 25-hydroxycholesterol on the activity of HMG-CoA reductase was determined. Human diploid fibroblasts (HDF) and the human melanoma cell line SK-MEL-2 converted 25-hydroxycholesterol into 7 alpha,25-dihydroxycholesterol and 7 alpha,25-dihydroxy-4-cholesten-3-one while the virus-transformed fibroblast line 90VA-VI, the colon carcinoma cell line WiDr and the breast cancer cell line MDA-231 did not express 7 alpha-hydroxylase activity. The 7 alpha-hydroxylation of 25-hydroxycholesterol in HDF could be stimulated by dexamethasone and cortisol and inhibited by metyrapone. An unidentified, possibly 4-hydroxylated, metabolite was formed by 90VA-VI cells and a polar, probably conjugated, metabolite was formed by WiDr cells. The 7 alpha-hydroxylated metabolites of 25-hydroxycholesterol suppressed the activity of HMG-CoA reductase to a similar extent as 25-hydroxycholesterol in HDF but not in 90VA-VI cells, while the 7 alpha-hydroxylated metabolites of 27-hydroxycholesterol suppressed the activity of HMG-CoA reductase also in 90VA-VI cells. The suppression of HMG-CoA reductase activity by 25- and 27-hydroxycholesterol was decreased or abolished by dehydroepiandrosterone or pregnenolone which have little or no effect on the 7 alpha-hydroxylation. The results indicate that 7 alpha-hydroxylation is not directly involved, positively or negatively, in the action of 25- or 27-hydroxycholesterol as suppressors of HMG-CoA reductase activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
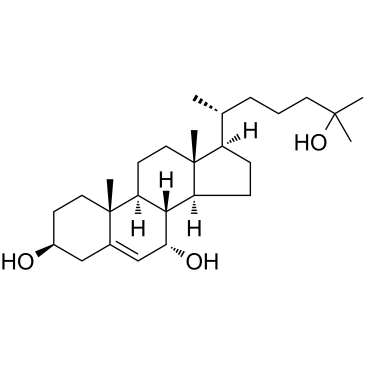 |
(3β,7α)-Cholest-5-ene-3,7,25-triol
CAS:64907-22-8 |
C27H46O3 |
|
Detection of dihydroxycholesterols in human plasma using HPL...
2015-07-01 [Steroids 99 , 131-8, (2015)] |
|
Molecular characterization of oxysterol binding to the Epste...
2012-10-12 [J. Biol. Chem. 287(42) , 35470-83, (2012)] |
|
Oxysterol gradient generation by lymphoid stromal cells guid...
2012-09-21 [Immunity 37(3) , 535-48, (2012)] |
|
Studies on the immunosuppressive properties of 7,25-dihydrox...
1988-01-01 [Int. J. Immunopharmacol. 10(5) , 511-8, (1988)] |
|
Oxysterols, but not cholesterol, inhibit human immunodeficie...
1998-11-01 [Antivir. Chem. Chemother. 9(6) , 491-6, (1998)] |