| Structure | Name/CAS No. | Articles |
|---|---|---|
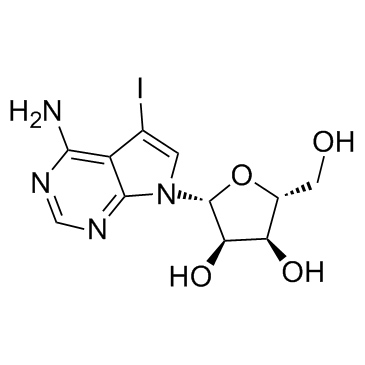 |
5-Iodotubercidin
CAS:24386-93-4 |
|
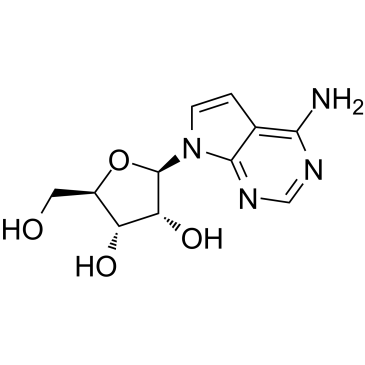 |
7-Deazaadenosine(Tubercidin)
CAS:69-33-0 |