| Structure | Name/CAS No. | Articles |
|---|---|---|
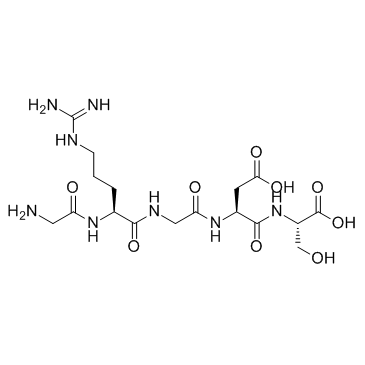 |
H-Gly-Arg-Gly-Asp-Ser-OH
CAS:96426-21-0 |
|
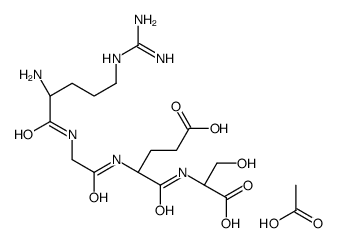 |
Arg-Gly-Glu-Ser acetate salt
CAS:159002-32-1 |