Bioorganic & Medicinal Chemistry Letters
2001-10-08
Synthesis, topoisomerase I inhibition and antitumor cytotoxicity of 2,2':6',2"-, 2,2':6',3"- and 2,2':6',4"-terpyridine derivatives.
L X Zhao, T S Kim, S H Ahn, T H Kim, E K Kim, W J Cho, H Choi, C S Lee, J A Kim, T C Jeong, C J Chang, E S Lee
Index: Bioorg. Med. Chem. Lett. 11(19) , 2659-62, (2001)
Full Text: HTML
Abstract
For the development of new anticancer agents, 2,2':6',2"-, 2,2':6',3"- and 2,2':6',4"-terpyridine derivatives were designed and evaluated for their topoisomerase I inhibitory activity and antitumor cytotoxicity. Structure-activity relationship studies indicated that 2,2':6',2"-terpyridine derivatives were highly cytotoxic toward several human tumor cell lines, whereas 2,2':6',3"- and 2,2':6',4"-terpyridine derivatives were potent topoisomerase I inhibitors.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
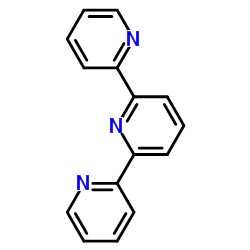 |
2,2':6',2''-TERPYRIDINE
CAS:1148-79-4 |
C15H11N3 |
Related Articles:
More...
|
Thousands of chemical starting points for antimalarial lead ...
2010-05-20 [Nature 465 , 305-10, (2010)] |
|
Metal complexes with superoxide dismutase-like activity as c...
2006-12-01 [Bioorg. Med. Chem. Lett. 16 , 5982-7, (2006)] |
|
In silico activity profiling reveals the mechanism of action...
2008-07-01 [Proc. Natl. Acad. Sci. U. S. A. 105 , 9059-64, (2008)] |
|
Rapid and sensitive amino-acid sequencing of cloning Thermus...
2004-01-01 [J. Proteome Res. 3(5) , 983-7, (2004)] |
|
Synthesis of 2,6-diaryl-substituted pyridines and their anti...
2008-04-01 [Eur. J. Med. Chem. 43 , 675-82, (2008)] |