Toxicokinetics, recovery, and metabolism of triclopyr butotyl (ACTP) ester in goats.
Tapas K Sar, Biplab Bagchi, Shyamal K Das, Tapan K Mandal, Animesh K Chakraborty, Anjan Bhattacharyya, Ashim Choudhury
Index: J. Agric. Food Chem. 50(15) , 4202-9, (2002)
Full Text: HTML
Abstract
Toxicokinetic behavior, recovery, and metabolism studies of ACTP ester and its effect on cytochrome P(450) content of liver microsomal pellet were carried out in black Bengal goat after a single intravenous administration of 11.88 mg kg(-1) and consecutive oral administration of 79.22 mg kg(-1) for 7 days. ACTP ester achieved a maximum blood concentration of 42.64 +/- 4.26 microg mL(-1) at 0.08 h after intravenous administration followed by a sharp decline until 0.5 h, and the minimum blood concentration was recorded at 36 h (1.93 +/- 0.14 microg mL(-1)) postdosing. The kinetic behavior of ACTP ester followed a "two-compartment open model". Comparatively shorter alpha (0.81 +/- 0.02 h(-1)) and greater t1/2 (alpha) (0.86 +/- 0.03 h) indicated a slower rate of distribution of ACTP ester in goat. The t1/2(beta)()) (14.83 +/- 1.49 h) and V(d(area)) (0.91 +/- 0.19 L kg(-1)) suggested a longer elimination phase with general distribution in all compartments of the body. The higher T/B and K12/K21 values associated with a lower f(c) value suggested longer persistence in the tissue compartment at higher concentration. The higher Cl(R) compared to Cl(H) indicated the major amount was eliminated by the kidney. Maximum concentration of ACTP ester including its metabolites, triclopyr acid and trichloropyridinol, was excreted through urine at 48 h. The recovery of ACTP ester including metabolites after repeated nontoxic oral dose administration was 70.09%, of which recovery from feces was 4.45%, suggesting the major portion of administered ACTP ester was absorbed through the gastrointestinal tract of the goat. All of the tissues contained ACTP ester and its metabolites. ACTP ester did not alter the cytochrome P(450) content of the liver tissue following repeated nontoxic oral dose administration for 7 days.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
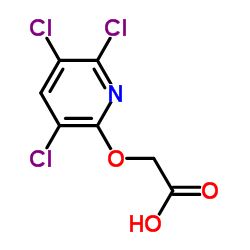 |
Triclopyr
CAS:55335-06-3 |
C7H4Cl3NO3 |
|
Are DNA-damaging effects induced by herbicide formulations (...
2014-10-01 [Aquat. Toxicol. 155 , 213-21, (2014)] |
|
Dissipation of four forest-use herbicides at high latitudes.
2008-10-01 [Environ. Sci. Pollut. Res. Int. 15(7) , 573-83, (2008)] |
|
Effects of herbicides on Behr's metalmark butterfly, a surro...
2012-05-01 [Environ. Pollut. 164 , 24-7, (2012)] |
|
Determination of chlorinated acid herbicides in vegetation a...
2007-01-01 [J. AOAC Int. 90(5) , 1402-10, (2007)] |
|
Clinical outcome of acute intoxication due to ingestion of a...
2011-11-01 [Clin. Toxicol. (Phila.) 49(9) , 815-9, (2011)] |