| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
HIV Protease Substrate IV
CAS:128340-47-6 |
|
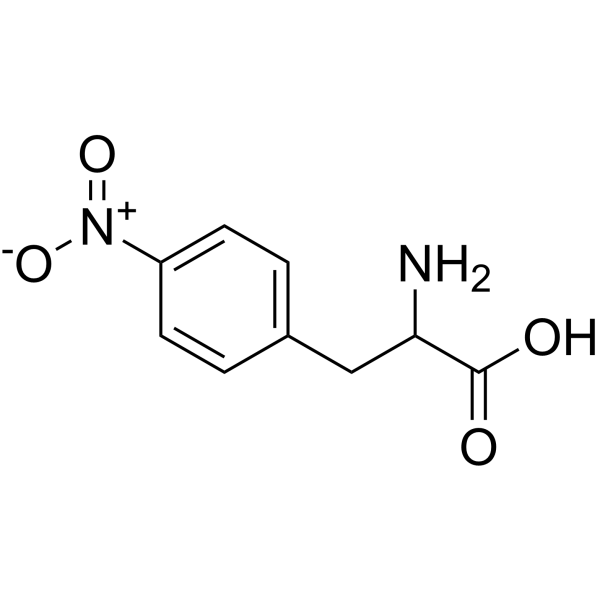 |
L-4-Nitrophenylalanine
CAS:2922-40-9 |