| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
AC-ALA-ARG-VAL-LEU-ALA-GLU-ALA-NH2
CAS:128340-47-6 |
|
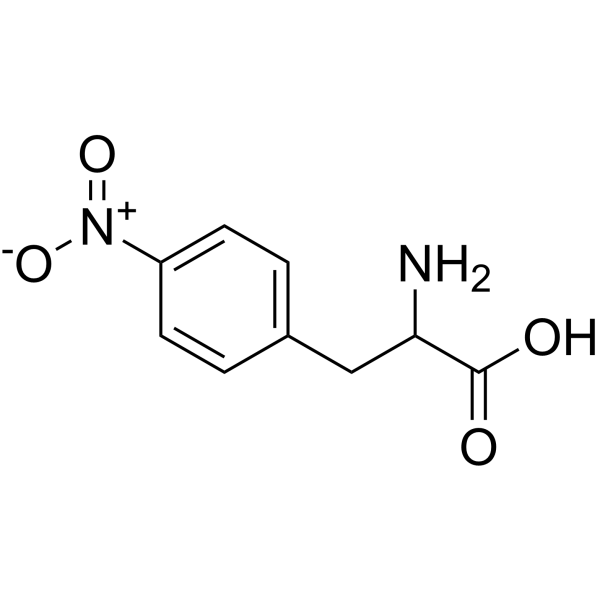 |
4-硝基-DL-苯丙氨酸
CAS:2922-40-9 |