| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(S)-(+)-4-Amino-3-hydroxybutyric acid
CAS:7013-05-0 |
|
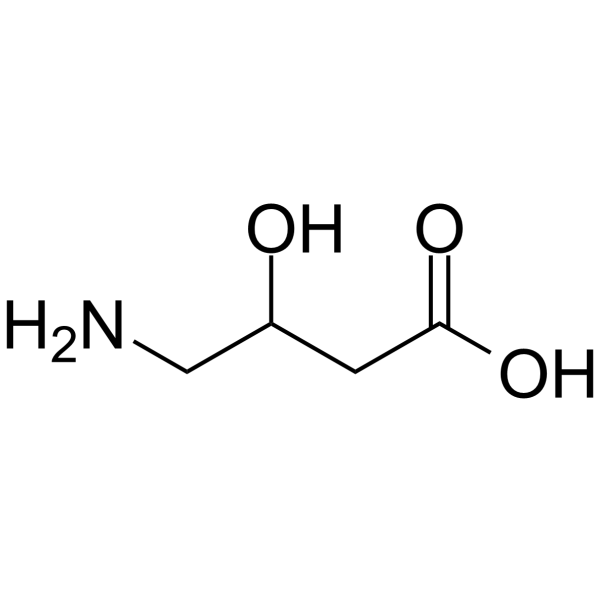 |
DL-γ-Amino-β-hydroxybutyric acid
CAS:924-49-2 |