Journal of Organic Chemistry
2000-08-25
A novel synthesis of racemic and enantiomeric forms of prostaglandin B1 methyl ester.
M Mikołajczyk, M Mikina, A Jankowiak
Index: J. Org. Chem. 65(17) , 5127-30, (2000)
Full Text: HTML
Abstract
3-(Dimethoxyphosphorylmethyl)cyclopent-2-enone was converted into (+/-)-prostaglandin B1 methyl ester in two steps involving regioselective alkylation at C(2) with methyl 7-iodoheptanoate and subsequent Horner-Wittig reaction with dimer of 2-hydroxyheptanal (42% overall yield). The use of (R)- and (S)-2-(tert-butyldimethylsilyloxy)heptanal for the Horner olefination reaction gave, after deprotection of the hydroxy group, the enantiopure forms of the title compound in 28% overall yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
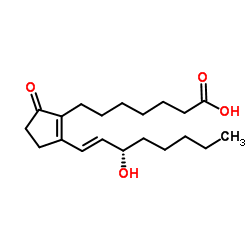 |
Prostaglandin B1
CAS:13345-51-2 |
C20H32O4 |
Related Articles:
More...
|
NADP(+)-dependent dehydrogenase activity of carbonyl reducta...
2015-06-01 [Free Radic. Biol. Med. 83 , 66-76, (2015)] |
|
Prostaglandin B1 can modify the pressor responses to sympath...
1983-09-01 [Res. Commun. Chem. Pathol. Pharmacol. 41(3) , 397-405, (1983)] |
|
Biophysical studies on molecular mechanism of abortificient ...
1982-02-21 [J. Theor. Biol. 94(4) , 943-9, (1982)] |
|
The effect of NaCl intake on 9-ketoprostaglandin reductase a...
1985-08-01 [Prostaglandins 30(2) , 335-49, (1985)] |
|
The percutaneous penetration of prostaglandin E1 and its alk...
1999-04-19 [J. Control. Release 58(3) , 349-55, (1999)] |