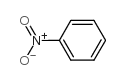
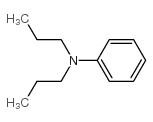
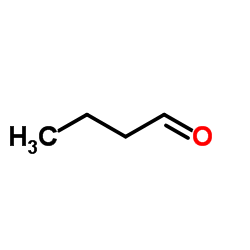
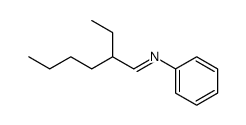
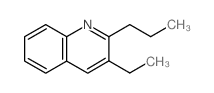
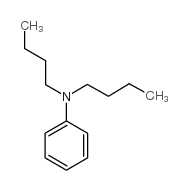
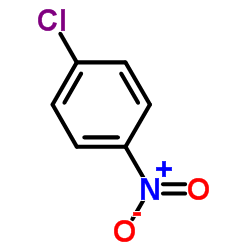
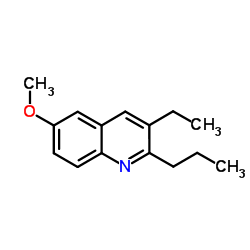
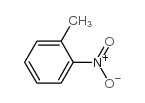
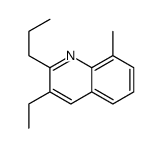
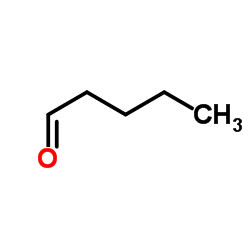
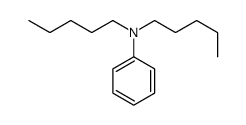
|
~59% |
|
~9% |
|
~55% |
|
~43% |
|
~12% |
|
~33% |
|
~36% |
|
~5% |