Role of the C-terminal domain in the structure and function of tetrameric sodium channels.
Claire Bagnéris, Paul G Decaen, Benjamin A Hall, Claire E Naylor, David E Clapham, Christopher W M Kay, B A Wallace
Index: Nat. Commun. 4 , 2465, (2013)
Full Text: HTML
Abstract
Voltage-gated sodium channels have essential roles in electrical signalling. Prokaryotic sodium channels are tetramers consisting of transmembrane (TM) voltage-sensing and pore domains, and a cytoplasmic carboxy-terminal domain. Previous crystal structures of bacterial sodium channels revealed the nature of their TM domains but not their C-terminal domains (CTDs). Here, using electron paramagnetic resonance (EPR) spectroscopy combined with molecular dynamics, we show that the CTD of the NavMs channel from Magnetococcus marinus includes a flexible region linking the TM domains to a four-helix coiled-coil bundle. A 2.9 Å resolution crystal structure of the NavMs pore indicates the position of the CTD, which is consistent with the EPR-derived structure. Functional analyses demonstrate that the coiled-coil domain couples inactivation with channel opening, and is enabled by negatively charged residues in the linker region. A mechanism for gating is proposed based on the structure, whereby splaying of the bottom of the pore is possible without requiring unravelling of the coiled-coil.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
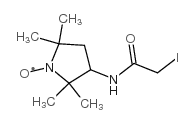 |
3-(2-Iodoacetamido)-PROXYL
CAS:27048-01-7 |
C10H18IN2O2 |
|
Characterization of ERM transactivation domain binding to th...
2015-08-18 [Nucleic Acids Res. 43 , 7110-21, (2015)] |
|
Functional role of the flexible N-terminal extension of FKBP...
2013-01-01 [Sci. Rep. 3 , 2985, (2013)] |
|
Escherichia coli antitoxin MazE as transcription factor: ins...
2015-01-01 [Nucleic Acids Res. 43(2) , 1241-56, (2015)] |
|
A fully enzymatic method for site-directed spin labeling of ...
2014-09-01 [Nucleic Acids Res. 42(15) , e117, (2014)] |
|
Spin labeled calmodulin: a new probe for studying Ca2+ and m...
1980-01-01 [Ann. N. Y. Acad. Sci. 356 , 20-32, (1980)] |