Functional role of the flexible N-terminal extension of FKBP38 in catalysis.
Congbao Kang, Hong Ye, Joel Chia, Bo-Hwa Choi, Sirano Dhe-Paganon, Bernd Simon, Ulrike Schütz, Michael Sattler, Ho Sup Yoon
Index: Sci. Rep. 3 , 2985, (2013)
Full Text: HTML
Abstract
FKBP38 regulates apoptosis through unique interactions with multiple regulators including Bcl-2. Interestingly, the peptidylprolyl isomerase activity of FKBP38 is only detectable when it binds to calcium-saturated calmodulin (CaM/Ca(2+)). This, in turn, permits the formation of a complex with Bcl-2. FKBP38 thereby provides an important link between isomerase activity and apoptotic pathways. Here, we show that the N-terminal extension (residues 1-32) preceding the catalytic domain of FKBP38 has an autoinhibitory activity. The core isomerase activity of FKBP38 is inhibited by transient interactions involving the flexible N-terminal extension that precedes the catalytic domain. Notably, CaM/Ca(2+) binds to this N-terminal extension and thereby releases the autoinhibitory contacts between the N-terminal extension and the catalytic domain, thus potentiating the isomerase activity of FKBP38. Our data demonstrate how CaM/Ca(2+) modulates the catalytic activity of FKBP38.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
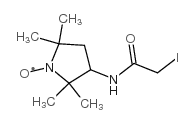 |
3-(2-Iodoacetamido)-PROXYL
CAS:27048-01-7 |
C10H18IN2O2 |
|
Characterization of ERM transactivation domain binding to th...
2015-08-18 [Nucleic Acids Res. 43 , 7110-21, (2015)] |
|
Escherichia coli antitoxin MazE as transcription factor: ins...
2015-01-01 [Nucleic Acids Res. 43(2) , 1241-56, (2015)] |
|
A fully enzymatic method for site-directed spin labeling of ...
2014-09-01 [Nucleic Acids Res. 42(15) , e117, (2014)] |
|
Role of the C-terminal domain in the structure and function ...
2013-01-01 [Nat. Commun. 4 , 2465, (2013)] |
|
Spin labeled calmodulin: a new probe for studying Ca2+ and m...
1980-01-01 [Ann. N. Y. Acad. Sci. 356 , 20-32, (1980)] |