Organic & Biomolecular Chemistry
2006-07-21
Synthesis of macrocyclic analogues of the neuroprotective agent glycyl-L-prolyl-L-glutamic acid (GPE).
Paul W R Harris, Margaret A Brimble
Index: Org. Biomol. Chem. 4(14) , 2696-709, (2006)
Full Text: HTML
Abstract
The syntheses of seven macrocyclic analogues of the neuroprotective tripeptide glycyl-L-prolyl-L-glutamic acid (GPE) are described. Macrocycles 6 and 7 mimic the cis conformer of GPE whereas macrocycles 2-5, 8, and 9 mimic the trans conformer of GPE. The macrocyclic peptides of well-defined geometry were prepared via Grubbs ring closing metathesis of an appropriate diene precursor. In turn each of the diene precursors were prepared from the readily available allyl-substituted amino acid building blocks 12, 13, 14, 27, 36 and 51.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
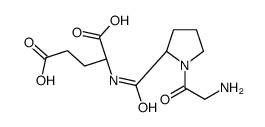 |
glycyl-prolyl-glutamic acid
CAS:32302-76-4 |
C12H19N3O6 |
Related Articles:
More...
|
Ethyl acetate extract from Glycosmis parva leaf induces apop...
2015-04-01 [Pharm. Biol. 53(4) , 540-7, (2015)] |
|
Analogues of the neuroprotective tripeptide Gly-Pro-Glu (GPE...
2005-05-02 [Bioorg. Med. Chem. Lett. 15 , 2279-83, (2005)] |
|
Phospholipids trigger Cryptococcus neoformans capsular enlar...
2011-05-01 [PLoS Pathog. 7 , e1002047, (2011)] |
|
N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH...
2000-03-24 [Brain Res. 859(2) , 286-92, (2000)] |
|
Neuroprotective effects of the N-terminal tripeptide of IGF-...
2001-12-13 [Brain Res. 922(1) , 42-50, (2001)] |