Bioorganic & Medicinal Chemistry
2005-01-17
Synthesis and neuroprotective activity of analogues of glycyl-L-prolyl-L-glutamic acid (GPE) modified at the alpha-carboxylic acid.
Nicholas S Trotter, Margaret A Brimble, Paul W R Harris, David J Callis, Frank Sieg
Index: Bioorg. Med. Chem. 13(2) , 501-17, (2005)
Full Text: HTML
Abstract
The synthesis of nine GPE* analogues, wherein the alpha-carboxylic acid group of glutamic acid has been modified, is described by coupling readily accessible N-benzyloxycarbonyl-glycyl-L-proline 2 with various analogues of glutamic acid. Pharmacological evaluation of the novel compounds was undertaken to further understand the role of the glutamate residue on the observed neuroprotective properties of the endogenous tripeptide GPE.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
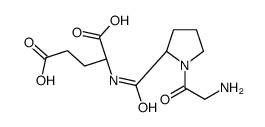 |
glycyl-prolyl-glutamic acid
CAS:32302-76-4 |
C12H19N3O6 |
Related Articles:
More...
|
Ethyl acetate extract from Glycosmis parva leaf induces apop...
2015-04-01 [Pharm. Biol. 53(4) , 540-7, (2015)] |
|
Analogues of the neuroprotective tripeptide Gly-Pro-Glu (GPE...
2005-05-02 [Bioorg. Med. Chem. Lett. 15 , 2279-83, (2005)] |
|
Phospholipids trigger Cryptococcus neoformans capsular enlar...
2011-05-01 [PLoS Pathog. 7 , e1002047, (2011)] |
|
N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH...
2000-03-24 [Brain Res. 859(2) , 286-92, (2000)] |
|
Neuroprotective effects of the N-terminal tripeptide of IGF-...
2001-12-13 [Brain Res. 922(1) , 42-50, (2001)] |