| Structure | Name/CAS No. | Articles |
|---|---|---|
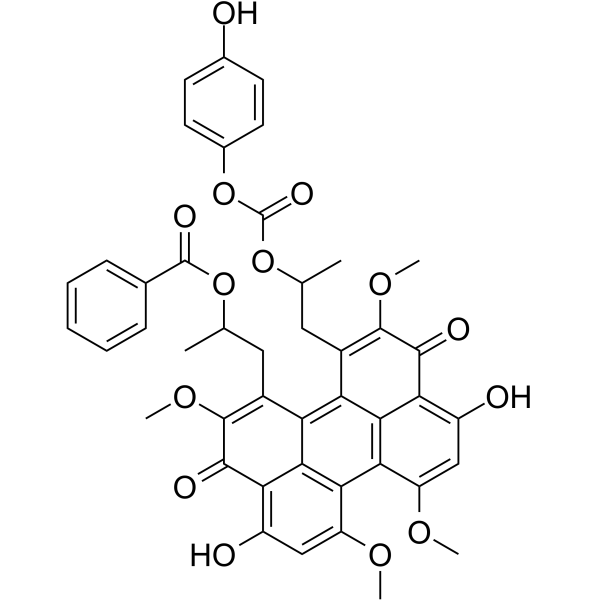 |
Calphostin C
CAS:121263-19-2 |
|
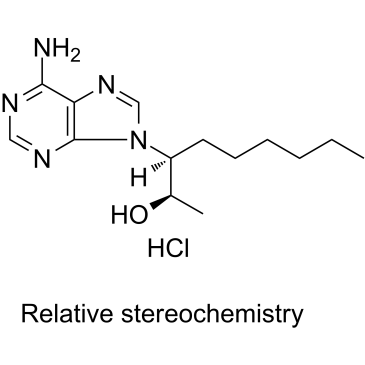 |
EHNA.HCl
CAS:58337-38-5 |