| Structure | Name/CAS No. | Articles |
|---|---|---|
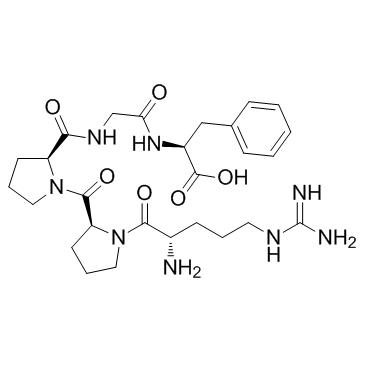 |
Bradykinin Fragment 1-5
CAS:23815-89-6 |
|
 |
H-Gly-Gly-Tyr-Arg-OH
CAS:70195-20-9 |
|
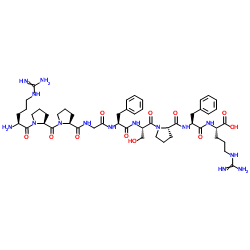 |
Bradykinin acetate salt
CAS:6846-03-3 |
|
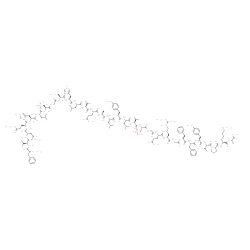 |
Insulin Chain B Oxidized
CAS:30003-72-6 |
|
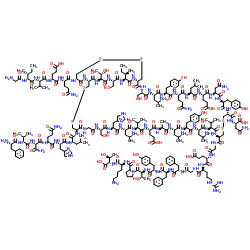 |
Insulin from porcine pancreas
CAS:12584-58-6 |