| Structure | Name/CAS No. | Articles |
|---|---|---|
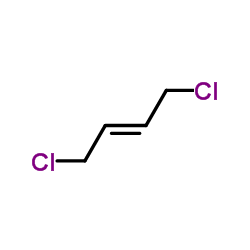 |
trans-1,4-Dichlorobutene
CAS:110-57-6 |
|
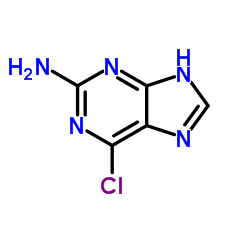 |
2-Amino-6-chloropurine
CAS:10310-21-1 |