Purification and characterization of pyridoxine 5'-phosphate phosphatase from Sinorhizobium meliloti.
Masaaki Tazoe, Keiko Ichikawa, Tatsuo Hoshino
Index: Biosci. Biotechnol. Biochem. 69(12) , 2277-84, (2005)
Full Text: HTML
Abstract
Here we report the purification and biochemical characterization of a pyridoxine 5'-phosphate phosphatase involved in the biosynthesis of pyridoxine in Sinorhizobium meliloti. The phosphatase was localized in the cytoplasm and purified to electrophoretic homogeneity by a combination of EDTA/lysozyme treatment and five chromatography steps. Gel-filtration chromatography with Sephacryl S-200 and SDS/PAGE demonstrated that the protein was a monomer with a molecular size of approximately 29 kDa. The protein required divalent metal ions for pyridoxine 5'-phosphate phosphatase activity, and specifically catalyzed the removal of Pi from pyridoxine and pyridoxal 5'-phosphates at physiological pH (about 7.5). It was inactive on pyridoxamine 5'-phosphate and other physiologically important phosphorylated compounds. The enzyme had the same Michaelis constant (K(m)) of 385 muM for pyridoxine and pyridoxal 5'-phosphates, but its specific constant [maximum velocity (V(max))/K(m)] was nearly 2.5 times higher for the former than for the latter.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
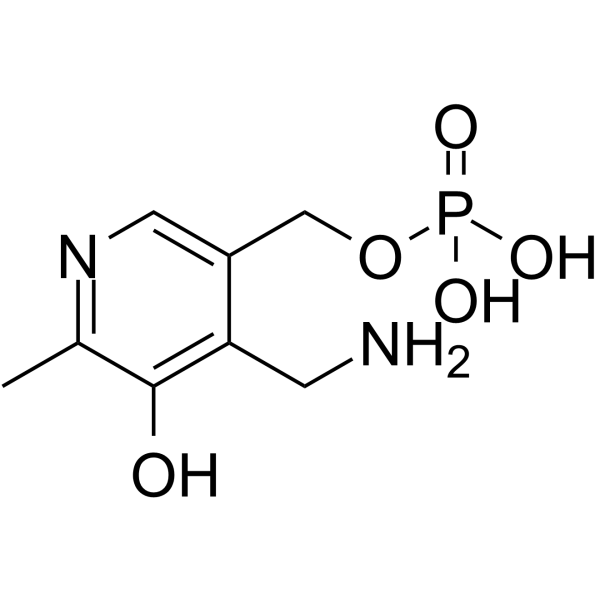 |
pyridoxamine phosphate
CAS:529-96-4 |
C8H13N2O5P |
|
Fungal recognition enhances mannose receptor shedding throug...
2011-03-11 [J. Biol. Chem. 286(10) , 7822-9, (2011)] |
|
The intestine plays a substantial role in human vitamin B6 m...
2013-01-01 [PLoS ONE 8(1) , e54113, (2013)] |
|
Interaction between glutamate dehydrogenase (GDH) and L-leuc...
2011-09-01 [Neurochem. Int. 59 , 518-524, (2011)] |
|
Crystal structures of the PLP- and PMP-bound forms of BtrR, ...
2006-10-01 [Proteins 65(1) , 220-30, (2006)] |
|
Cofactor-directed reversible denaturation pathways: the cofa...
2007-05-15 [Biochemistry 46(19) , 5819-29, (2007)] |