Cofactor-directed reversible denaturation pathways: the cofactor-stabilized Escherichia coli aspartate aminotransferase homodimer unfolds through a pathway that differs from that of the apoenzyme.
Edgar Deu, Jack F Kirsch
Index: Biochemistry 46(19) , 5819-29, (2007)
Full Text: HTML
Abstract
While the urea-mediated unfolding pathway of the Escherichia coli aspartate aminotransferase (eAATase) homodimer proceeds through a reversible three-state process with a partially folded dimeric intermediate, D D* 2U (E. Deu and J. F. Kirsch, accompanying paper), that of a cofactor-stabilized form differs. Pyridoxal phosphate, which binds at the intersubunit active sites, stabilizes the native form by 6 kcal mol-1 and dissociates during the D <==> D* transition. Reductive trapping of the cofactor to a nondissociable derivative (PPL-eAATase) precludes the formation of D*. A novel monomeric intermediate (M'-PPL) with 70% of the native secondary structure (circular dichroism) was identified in the unfolding pathway of PPL-eAATase: D-PPL2 <==> 2M'-PPL <==> 2U-PPL. The combined results define two structural regions with distinct stabilities: the active site region (ASR) and the generally more stable, dimerization region (DMR). The DMR includes the key intersubunit contacts. It is responsible for the multimeric nature of D*, and its disorder leads to dimer dissociation. Selective strengthening of the ASR-cofactor interactions by cofactor trapping reverses the relative stabilities of the two regions (from DMR > ASR in the apoenzyme to ASR > DMR in PPL-eAATase) and results in a reordering of the eAATase denaturation pathway.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
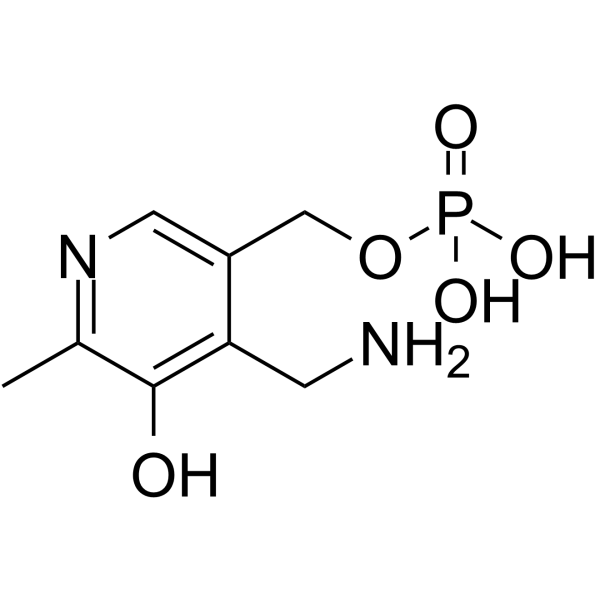 |
pyridoxamine phosphate
CAS:529-96-4 |
C8H13N2O5P |
|
Fungal recognition enhances mannose receptor shedding throug...
2011-03-11 [J. Biol. Chem. 286(10) , 7822-9, (2011)] |
|
The intestine plays a substantial role in human vitamin B6 m...
2013-01-01 [PLoS ONE 8(1) , e54113, (2013)] |
|
Interaction between glutamate dehydrogenase (GDH) and L-leuc...
2011-09-01 [Neurochem. Int. 59 , 518-524, (2011)] |
|
Crystal structures of the PLP- and PMP-bound forms of BtrR, ...
2006-10-01 [Proteins 65(1) , 220-30, (2006)] |
|
Insights into the mechanism of oxidative deamination catalyz...
2008-07-08 [Biochemistry 47(27) , 7187-95, (2008)] |