| Structure | Name/CAS No. | Articles |
|---|---|---|
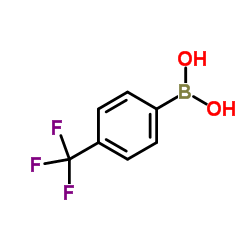 |
4-Trifluoromethylphenylboronic acid
CAS:128796-39-4 |
|
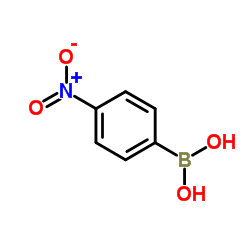 |
(4-Nitrophenyl)boronic acid
CAS:24067-17-2 |
|
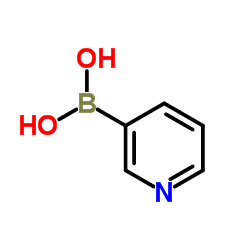 |
3-Pyridylboronic acid
CAS:1692-25-7 |
|
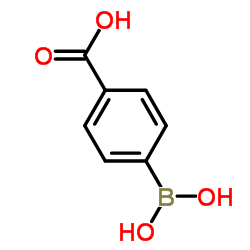 |
p-Boronobenzoic acid
CAS:14047-29-1 |